Internationale Partnersuche
Innovation & Technologie Angebot
Wearable assistive mobility exoskeletons that will be affordable in non-medical markets
Country of Origin: United Kingdom
Reference Number: TOUK20190726002
Publication Date: 26 July 2019
Summary
A UK company is bringing to market a core wearable assistive exoskeletons technology, starting from non-medical applications. Win-win partnerships are sought with end-users and developers, to adjust the existing prototype for existing applications, and find new ones (may include medical). It is designed to be affordable and make a business case. Dependent on route to market, the co-operation agreement may be in the form of licensing, research or technical co-operation, joint ventures.
Description
A small UK company is capitalising its know-how in exoskeletons. It was founded by researchers who have led a number of national R&D projects in the UK, Sweden and India, since 2011. The result of these projects is said exoskeletons are at TRL 6-7 and have been tested on elderly persons (see the Pictures). The researchers have also been leading ISO/IEC robot safety standardisation projects for the new types of service robots in non-medical and medical sectors under ISO TC299 and IEC 62A.
Recently, companies have been formed in the UK and India to develop wearable mobility assistive exoskeletons for supporting elderly persons to perform normal daily living activities, thereby improving quality of life and at the same time reducing healthcare costs. The subsidiary in India contributes towards manufacturing them in a commercially viable way. Non-medical exoskeleton products are formally defined as physical assistant robots in ISO 13482 (published in 2014), and medical exoskeletons are defined as medical robots for rehabilitation, assessment, compensation or alleviation (RACA robots) in IEC 80601-2-78 (published in 2019). The non-medical products will be launched in a foreseeable future, with the medical ones to follow later. The commercialisation activities are being supported by key organisations in China, South Korea, Taiwan, UK and USA to realise a supply chain of modular components and processes for commercialising wearable exoskeletons.
Applications
• Non-medical: Assistive products for healthy elderly persons to perform basic mobility motions for daily living. These include providing stability during standing, physical assistance for sit-to-stand/ stand-to-sit transfers, support for straight walking, stair ascending/ descending, etc.
• Non-medical: Assistive products for workers to perform physically demanding tasks
• Medical: RACA devices for patient with medical physical impairments
As this is a platform technology, the UK company is seeking both end-users and development partners. The initial systems need to be tested further and possibly adjusted. The type of co-operation may vary and can include technical co-operation, licensing, joint venture. Licensing may go both ways, dependent on the agreed subtasks agreed and IP resulting thereof. Technical co-operation is sought for further modifications, with both end-users and other technology developers. For manufacturing, a JV may be suitable with developers, or larger end-users. End-users and developers may find new or specific applications, and jointly create new products under technical and research co-operation. If the development stays largely in the UK, a joint venture may suit manufacturing and a commercial launch.
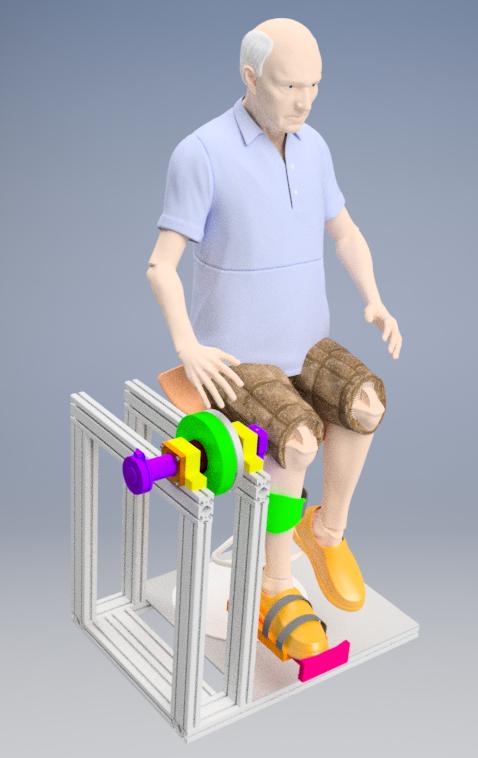



Advantages and Innovations
The exoskeletons on the market are very bespoke and expensive and being made in very low volumes.
The new technology has been designed for cost so that it will make a business case in non-medical segments. It is sufficiently close to market:
• The core wearable technology has already been developed and demonstrated in R&D projects;
• Detailed knowledge of ISO safety regulations in new emerging assistive exoskeleton sectors (medical and non-medical sectors);
• Cooperation with Indian partnership initiated for developing initial simplifications to technology and realisation of commercially viable products;
• Support from international key players for developing component supply chains and testing prototype products.
Stage Of Development
Prototype available for demonstration
Stage Of Development Comment
Several advanced lower-body exoskeleton prototypes have been developed and tested by able researchers and elderly persons via ethical approval awarded by a Regional Board in Sweden.
A technical team has been set up in India and is being supported by Punjab State Government via Punjab Invest and being supported by technical team in UK and other countries. The team is developing the detailed refinements to key components to realise initial assistive exoskeleton products for healthy elderly persons. The targeted technical work is expected to conclude with the first prototype product in Q2 2020. Partnerships/collaborations are sought with suitable organisations to broaden the technology developments to other non-medical and medical sectors.
Requested partner
Specialised exoskeleton products for supporting healthy elderly users in daily living applications are already being developed. The assistive wearable technology and knowhow is offered to enterprises active in this and other application sectors for creating win-win partnerships able to test the initial systems and increase potential for commercialising as well as developing dedicated products to meet specific needs in other application domains. Such applications domains can include for example healthcare and industrial sectors, logistics, medical equipment such as RACA devices.
Cooperation offer ist closed for requests

