Internationale Partnersuche
Innovation & Technologie Angebot
A Korean company specialised in in-vitro diagnostics is looking for partners, interested in developing and licensing, commercialising aptamer immuno-PCR technology for in-vitro diagnostics of prostate cancer.
Country of Origin: South Korea
Reference Number: TOKR20200506001
Publication Date: 12 May 2020
Summary
A Korean company specialised in in-vitro diagnostics is looking for partners, interested in developing and commercialising aptamer immuno-PCR technology. This technology can detect not only prostate cancer but other types of cancers with 1000 times more sensitivity than the existing test technology, ELISA (Enzyme-Linked Immunosorbent Assay). The company prefers partners specialised in cancer diagnostics and in-vitro diagnostic kits under commercial, license, or research cooperation agreement.
Description
For the early diagnosis of prostate cancer, PSA (Prostate-Specific Antigen) test is primarily used. The test measures the level of PSA in a man’s blood by using ELISA. However, the PSA ELISA test has been reported to have low sensitivity and specificity. To overcome the limitation of conventional PSA ELISA test, the Korean company specialised in in-vitro diagnostics including Covid-19 real-time RT-PCT kit has developed the aptamer immuno-PCR technology.
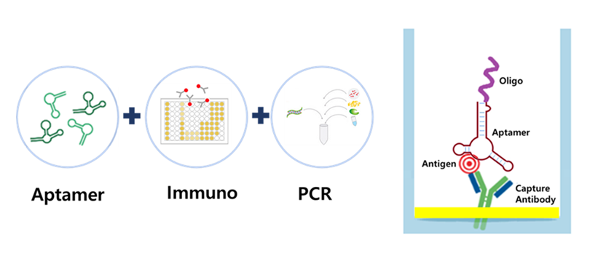
The technology is a modification of conventional immuno-PCR which combines ELISA and PCR to detect and quantify protein in clinical samples. Aptamers are oligonucleotide molecules that bind to a specific target molecule. Aptamer immuno-PCR here utilizes antibody-aptamer hybrid detection strategy in which aptamer replaces the detection antibody. Aptamer oligonucleotides with PCR amplification sequence are able to be amplified with real-time PCR for the quantification of initial amounts of target protein binding to capturing antibody.
The company is now developing a diagnostic kit for prostate cancer with the aptamer immuno-PCR, enabling to detect and quantify three different target proteins at the same time in one tube including PSA. Preliminary studies have shown that the simultaneous detection of multiple proteins is possible with this technology. Importantly, the test with the aptamer immuno-PCR has shown that the detection of PSA is 1,000 times more sensitive than ELISA. Additionally, one of the advantages of the method is that both urine samples and serum samples can be used for the diagnosis of prostate cancer for easy collection of clinical samples and convenient use in clinical laboratories.
The offered aptamer immuno-PCR technology is not only used in the diagnosis of prostate cancer but also applicable to other kinds of cancers. This diagnostic technology improves the analytical sensitivity and multiple detections of target proteins for cancer diagnosis. The cutting-edge technology is also useful to develop diagnostic kits of various diseases with novel protein biomarkers.
The company is now looking for collaborations with R&D institute or company dealing with cancer diagnostics and in-vitro diagnostic kits under a commercial agreement with technical assistance, license agreement or research cooperation agreement.
Advantages and Innovations
The offered technology is able to detect and quantify the protein antigen in urine for the diagnosis of prostate cancer and combined the advantages of ELISA (Enzyme-Linked Immunosorbent Assay) and real-time PCR can detect a low amount of protein in clinical samples.
The aptamer immuno-PCR is 1000 times more sensitive than conventional ELISA test and can detect multiplex proteins simultaneously. It means that multiple protein antigens are able to be detected and quantified in a single test from infants/toddler to elderly patients. Additionally, it could be applied to diagnosis of various cancers using various clinical samples such as serum and urine.
Stage Of Development
Under development/lab tested
Requested partner
The Korean company prefers R&D institution or company, dealing with cancer diagnostics and in-vitro diagnostic kits and interested in collaboration in the further development and commercialization of diagnostics kits under a commercial agreement with technical assistance, license agreement or research cooperation agreement.
Cooperation offer ist closed for requests

