Internationale Partnersuche
Innovation & Technologie Angebot
Peripheral nerve regeneration using natural protein nanofibers to be transfered from a French technology transfer office
Country of Origin: France
Reference Number: TOFR20180903001
Publication Date: 5 September 2018
Summary
Functionalized natural protein nanofibers have been validated for use as a scaffold for nerve regeneration, by a research lab within the Paris region. The French Technology Transfer Office (TTO) acting on behalf the research lab, is looking for a licensing agreement or a technical cooperation agreement with biomedical companies operating in medical devices and tissue engineering.
Description
A French technology transfer Office (TTO) is acting on behalf many major life science research labs in the Paris region. One of them succeeded in setting up an innovative medical device to be used as a scaffold for peripheral nerve regeneration.
* Market challenges :
Post-traumatic nerve damages following traffic accident or any violent shock or impact need nerve regeneration. The US market of peripheral nerve breakdown has been estimated around 1,7 billions $ in 2016.
Apart from the use of autograft within a short time after the nerve has been cut, there is still no synthetic substitute which has been proved to be efficient in nerve regeneration. Indeed, only one competitor has been identified without any relevant data demonstrating its regeneration effect. The main challenge is to set up a biocompatible scaffold leading to uni-directional nerve regeneration guidance and significant mechanical strength of the reconstructed nervous tissue.
* State of the art :
The reference practice is the use of autograft. Unfortunately, it strongly depends on the availability of nervous segments within the same subject and the surgery for nerve tissue extraction and grafting is quite complex.The only existing competitor doesn't give any information about the nature of the synthetic materiel used as a nerve substitute, and no results have been published about its performances.
* Proposed technology :
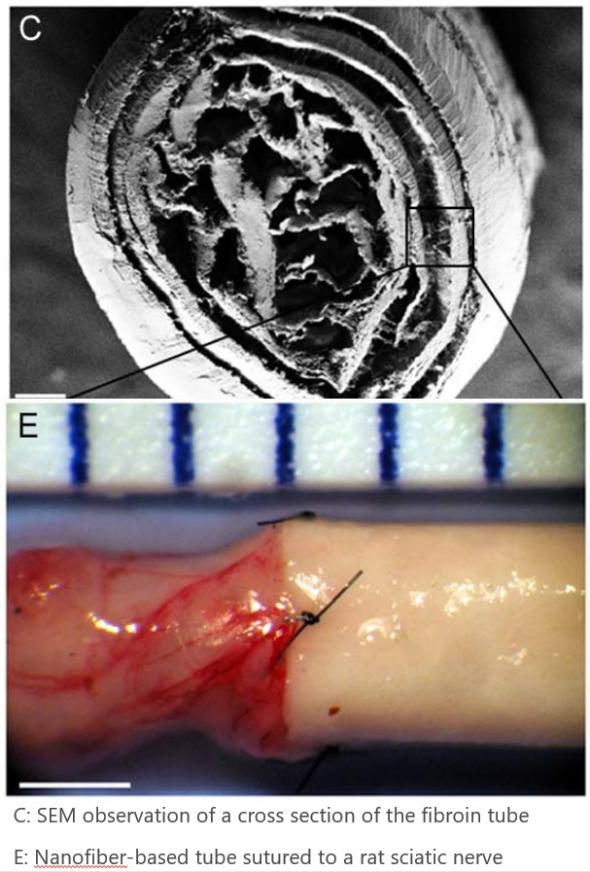
An innovative scaffold for nerve regeneration has been set up using functionalized natural silk. First, electric spinning process is used to produce biomimetic silk nanofibers to be used as nervous fibers. Then, functionalization is performed by means of growth factors.
Both in vitro an in vivo testing (small animal model) have been successfull when applied to the rat sciatic nerve. It has been assessed by means of morphologic studies using scanning electron microscopy (MES), and mechanical tensile tests.
The French TTO is looking for companies operating in medical devices or biomaterials sector. Expected outcomes can be either a technical cooperation agreement or a licensing agreement. Especially, a technical cooperation program can be partially funded by the TTO in order to assess prototype regeneration performances.
* Keywords :
peripheral nervous system, nerve guidance, nerve regeneration,silk.

Advantages and Innovations
The proposed medical device is made of natural silk in addition with specific molecules (no drugs, more information using non-disclosure agreement). As compared to the only identified competitor, it shows a strong axial guidance for peripheral nerve regeneration and an increased mechanical strength.
* Advantages :
- The natural silk shows an excellent biocompatibility,
- silk fibroins mechanical strength has been proved to be high enough during suturing process (tested in real surgical conditions),
- silk fibroins can be shaped into many various formats with repect to neurosurgical needs,
- natural silk can be readily available.
Stage Of Development
Under development/lab tested
Stage Of Development Comment
In vitro proof of concept has been successfully performed on rat neuron cultures.
In vivo proof of concept has been successfully performed on rat sciatic nerve.
The resulting nervous material has been morphologically assessed using scanning electron microscopy, and its mechanical properties have been validated from mechanical tensile testing.
Requested partner
The French TTO is looking for companies operating in medical devices or biomaterials sector, interested in innovative tissue engineering solutions.
Expected outcomes can be either a technical cooperation agreement or a licensing agreement. Especially, a technical cooperation program can be partially funded by the TTO in order to assess prototype regeneration performances.
Cooperation offer ist closed for requests

