Internationale Partnersuche
Innovation & Technologie Angebot
Silicone device for cellularized membrane culture offered under licence agreement
Country of Origin: Spain
Reference Number: TOES20191106001
Publication Date: 6 November 2019
Summary
Basque R&D+I centre specialized in food and polymer technology is looking for licensees for a medical device they have developed together with a University Hospital of reference in Navarre (north of Spain). A European patent application has been filed for the device aimed at the culture of cellularized membranes.
Description
This technological centre is a member of the science & technology Basque network. It has been dealing with polymer technology for over 12 years. The specialization areas and key elements are four:
1) Lightweight and material substitution. Dynamic and impact testing machinery.
2) Simulation of manufacturing processes and structural behaviour.
3) Fatigue in elastomers; prediction of rubber components’ durability.
4) Medical devices: design, manufacture and test medical devices for cell therapy based in polymer materials. An ISO 7 cleanroom with Silicone and thermoplastic injection molding technology.
As for the University Hospital, it has a dedicated GMP (good manufacturing practice) cell laboratory and a wide experience in cell culture for clinical applications.
The main features of the technology are the following:
This device is used for culture cellularized membranes for their use in biomedical research or their use as advanced therapy medicaments. It can be used effectively with all types of CO2 incubators for cell culture and different kinds of membranes.
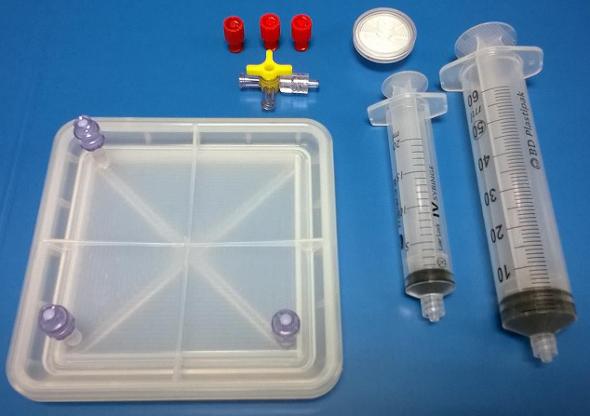
The culture device consists of a base and a self-assembling lid that generates a hermetically closed chamber. The lid is provided with one, or more access ports for accessing to the culture vessel. The access ports are configured as truncoconical sockets in which two Luer-Lock valves (commercial) are assembled. These valves could be connected to syringes to insert in the vessel the cells and the culture media in a safe and clean manner, without contaminating the space formed in the interior of the vessel. Other elements such as e.g. a filter may be connected to the corresponding access port to allow the gaseous interchange. The sidewall of the lid presses the membrane against the bottom surface of the culture vessel to prevent the membrane from moving or shrinking and thus the cells may properly adhere to the membrane and grow.
The biocompatible material with which this prototype has been obtained is an elastic polymer, that allows to withstand high internal pressures which provides one of its main advantages; the hermetic closure. Moreover, the device is manufactured with a biocompatible, sterilizable polymeric material, which allows its use in pharmaceutical GMP conditions and the possibility of certification as a medical device.
The potential applications are dermatology, cardiology, urology and ophthalmology among others.
Once the desired cell growth is obtained, the culture can be prepared and sent where it will be implanted (for example) without the possibility of losing the culture, orr the sterile conditions.
This container is the result of teaming up with a University Hospital from Navarre who provided knowledge regarding the requirements and optimization needs of its clinical practice. On the other hand, the research centre catered for the know-how in the design and development of medical devices. At present, the university and the centre are tackling an Interreg-Poctefa and an H2020 project together.
The type of collaboration that best suits both organizations is that of a licencing agreement on the device with any possible partner worldwide.



Advantages and Innovations
The device for culture cellularized membranes has as main advantages over the rest of its competitors that:
1) provides simplicity and functionality,
2) can be closed tightly (providing a proper environment but with an easy access)
3) allows immobilization of the membrane easily
4) can be used for sterile transportation of the cellularized membranes to the operation room
In the current market there is no hermetic device in which it is possible to grow the cells without the probability of contamination neither a device that is capable of supporting the membrane to maintain uniformity and that can fix the membrane for transport to the place of use without problems.
It provides safety and efficacy in the cell culture and ability to fix membranes in an easy way. It is a culture vessel in a closed environment but with an easy access, so that it can be used for culture and as final packaging in the transport of the cellularized membrane to the operation room in a sterile and GMP conditions.
Stage Of Development
Concept stage
Stage Of Development Comment
Prototype manufactured
Preliminary proof of concept in a limited number of in vitro/in vivo trials
European patent application filed
Requested partner
The partner sought could be:
- companies that commercialize and distribute equipment and instruments for cell culture.
- companies that work with LSR (liquid silicone rubber) silicone and want to diversify their portfolio
The final users could be a researcher involved in any of the biotechnological groups or companies related with emerging therapies such as: gene therapy, somatic cell therapy, and tissue engineering.
The general conditions of the licencing agreement are open for negotiation and subject to change according to the type of partner that will be contacted.
Cooperation offer ist closed for requests

