Internationale Partnersuche
Innovation & Technologie Angebot
Novel self-expanding polyurethane foams intended for inert filling of pleural and other human cavities.
Country of Origin: Spain
Reference Number: TOES20170328002
Publication Date: 29 March 2017
Summary
A Spanish university has developed a new polymeric foaming material for in-situ filling and sealing of internal irregular different shaped human cavities. It can be self-expanded and self-moulded for avoiding complications in open internal cavities caused by infections, blooding, fistulae, dyspnoea or sepsis. It is easily applicable, safe and avoids the use of current aggressive treatments in pleural cavities. Companies are sought for licensing or technical cooperation agreements.
Description
The pleural cavity contains a minimal amount of liquid to maintain the negative pressure needed for an adequate pulmonary function. Several pathologies of the pleural cavity produce air or biological liquids (pus, blood, pleural openings, etc.) that are commonly solved by draining. However, some pathologies cause chronification leading to severe clinical complications such as infections, sepsis, fistulae, pain, haemorrhages or dyspnoeas.
The current treatments for draining the pleural cavities are mainly decortications, toracoplasties, thoracotomies, pedicular inserts, thoracic openings, and filling with different synthetic materials, which are aggressive and may cause severe clinical complications such as infections of fistulae. Copolymers of lactic acid and caprolactone have also been used but they cannot be injected throughout small cavities because they are not self-expandable and show insufficient mechanical properties.
Currently, several polyurethane foams intended for biomedical applications have been developed following two different approaches:
1. Biocompatible polyurethane foams containing different functional compounds for imparting self-adhesion properties.
2. Mixing of thermoplastic polyurethanes and biodegradable polyesters derived from polilactic acid able to produce closed-cell foams with good contraction and biocompatibility.
A Spanish research group has developed a new polymeric foaming material for in-situ filling and sealing of internal irregular different shaped human cavities, intended for patients suffering chronic pleural cavities and in-field injuries caused by bullets or accidental event leaving open blood vessels.
The new polymeric foams show a solid and a gas phase dispersed into the polymeric matrix, forming discrete or interconnected cells. The formation of in-situ polyurethane foams is reached by mixing two liquid components that can be injected by means of a double-container syringe. The two liquids components may have different compositions:
-Component 1: it may contain water, polyols, foaming agents, catalysts, surfactants, other polymers, plasticizers, inorganic and/or organic fillers, processing aids, rheological additives, radiological tracers and antibiotics, among other.
-Component 2: it contains isocyanate or isocyanates mixture.
Both components are liquid at room temperature and they can be mixed in few seconds before injecting the mixture in a syringe or in a dosing device to its deposition in liquid state in the internal human cavity (pleural cavity, blooding vessel, injury in the field). Thus, the foaming can be controlled for being fast or slow (less than one minute in any case). The composition is designed to start the foaming during the application of the two liquid components avoiding that the liquids can be in contact with the tissues surrounding the cavity.
The component 1 can be homogenized by mechanical stirring in less than one minute. Since the component 2 is liquid, it can be easily manipulated. The two liquid components can be mixed by mechanical stirring in less than 20 seconds. By changing the ratio of the two components, the foaming process can be controlled to be adapted to the specific cavity and to different geometries, shapes and size of the cavities.
They can be formulated for rending flexible, semi-rigid or rigid foams. In all cases a homogeneous size and cell distribution is obtained and all of them show adequate mechanical resistance.
The present invention can be applied in biomedical and veterinary sectors for the filling of internal cavities. It can be also used in accidental and in-field lesions or injuries causing blood bleeding.
The research group is looking for companies interested in acquiring this invention for commercial exploitation through licensing agreement or technical cooperation (Development of new applications, adaptation to specific needs of the company, technical reports and scientific assessment, etc

Advantages and Innovations
The new polyurethane foam is intended for filling internal human or animal cavities having different shapes and sizes. The most innovative aspect is the fact that they are self-expandable and self-modelled.
The main advantages of the novel developed foam are:
• Easy to apply, even though very small orifices.
• No adhesion to surrounding tissues.
• Very low density (i.e. low weight).
• It can be easily removed from the internal cavity, if necessary.
• Biocompatible and low risk of toxicity, carcinogenesis and local clinical complications.
• It can be dosed into open or closed cavities.
• It can be applied in the presence of blood or biological liquids.
• The resulting foams do not deteriorate over time and are stable.
• It does not cause infections.
• It does not affect the healing. Fistulae formation is not favoured.
• It does not interfere the heart or diaphragm movements.
• It is impermeable to biological fluids, water and blood.
• It has haemostatic effect.
• It allows the sealing of parenchyma or bronchial fistulae.
Stage Of Development
Under development/lab tested
Stage Of Development Comment
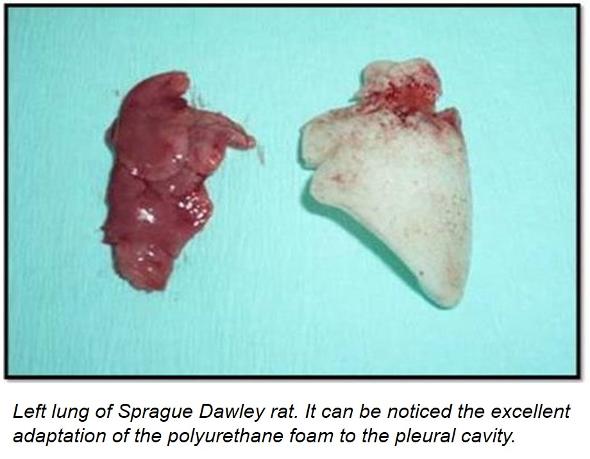
The technology has been developed at laboratory scale. A proof of concept has been tested for determining the viability of the new polyurethane foam in post-pneumonectomy cavity in 20 Sprague Dawley rats and 6 rabbits. The analysis of the extracted polyurethane foams shows the following evidences:
• The foams filled completely the cavity and they were adapted perfectly to the pleural cavity of the animals.
• The mechanical integrity of the foams was maintained and it was excellent.
• Ipsilateral displacement was not observed.
Requested partner
- Type of partner sought: Companies
- Specific area of activity of the partner: medical, biomedical, veterinary
- Task to be performed: Acquisition of this technology through license agreement. Technical cooperation agreements through:
• Joint development of new applications,
• Adaptation to the company’s needs,
• Technical reports and scientific assessment.
• Specific training
• Standardization services, calibration, national and international technical rules, etc.
• Staff exchange for specific periods of time (to learn specific techniques).
• Rent the internal equipment to clients that wish to continue their own tests.
Cooperation offer ist closed for requests

