Internationale Partnersuche
Innovation & Technologie Angebot
A 3D-printed biomimetic tumour angiogenesis model
Country of Origin: Germany
Reference Number: TODE20211014002
Publication Date: 14 October 2021
Summary
A German university offers a model that can be used to analyse tumour growth, tumour angiogenesis and therapeutic effects on a 3D-printed cell model. No animal tissue is needed. Therefore, this 3D-printed tumour model represents an ideal in-vitro-prescreening system for testing pharmaceuticals and diagnostics and might be able to circumvent or at least reduce the number of animal tests in drug development. Licensees are sought.
Description
Despite major progress in finding alternative methods to animal use in drug discovery and development, laboratory animal testing is in many instances still inevitable. The law also requires animal testing to determine the safety and efficacy of new medicines before testing in humans. To reduce the number of experimental animals, several in-vitro-screening systems have been developed using different 3D-in-vitro-tissue-engineering approaches. However, even though significant progress has been made, there is to date still no satisfying in-vitro-screening system available, which might represent a suitable alternative to animal studies.
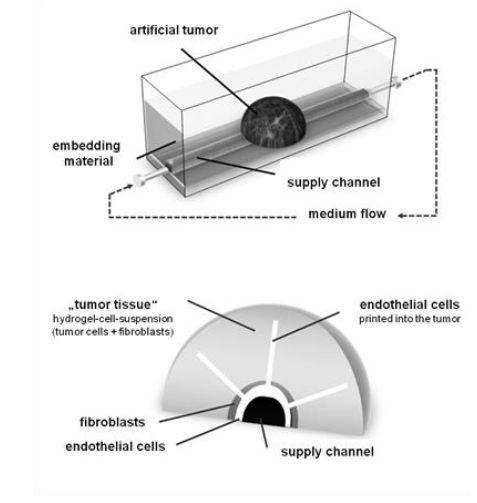
A German university is working on a new solution: Here 3D-printed structures are produced to generate a 3D-biomimetic tumour-angio¬genesis model. In a first step, a supply channel covered by an endothelial cell layer is generated using the 3D-bioprinting process and in a second step, tumour cells are embedded into a surrounding hydrogel-matrix cell-suspension.
Then, angiogenesis will start from the supply channel towards the tumour cells (which release angiogenic factors) leading to a vascularized and perfusable tumour.
This model can be used to analyse tumour growth, tumour angiogenesis and therapeutic effects on the 3D-printed cell model. This 3D-tumour model can be designed from pure biologic material (hydrogels and cells) without the need of synthetic development processes. No animal tissue is needed. Therefore, this 3D-printed Tumour Model represents an ideal in-vitro-prescreening system for testing pharmaceuticals and diagnostics and might be able to circumvent or at least reduce the number of animal tests in drug development.
Licensees from pharmaceutical and biotechnology industry are sought to use the invention commercially.
Advantages and Innovations
This is a novel in-vitro-screening system for
• Pre-screening of diagnostics and pharmaceuticals
• testing drugs during tumour therapy
• analysis of tumour growth and tumour angiogenesis
The process is furthermore more precise than other attempted solutions and it is replicable. It represents a new alternative to animal testing.
Stage Of Development
Under development/lab tested
Stage Of Development Comment
The technology has been validated in relevant environment.
Technology readiness level (TRL): 5
Requested partner
The university is offering access for commercial use in terms of a license agreement. Potential licensees are biotechnology industry and pharmaceutical industry interested in the new strategies in the 3D-cell-printing of tumour models and alternatives to animal testing.
Kooperationsanfrage stellen

