Internationale Partnersuche
Innovation & Technologie Angebot
Spray freeze-drying for pharmaceutical applications
Country of Origin: Germany
Reference Number: TODE20210304002
Publication Date: 4 March 2021
Summary
A German university offers an improved process for the spray freeze-drying of lipophilic compounds. As a result the powder made from these compounds is characterized by better flow-properties and more suitable for drug formulations. It also opens the possibility for various types of drug delivery. Partners from the pharmaceutical industry are sought for license agreements.
Description
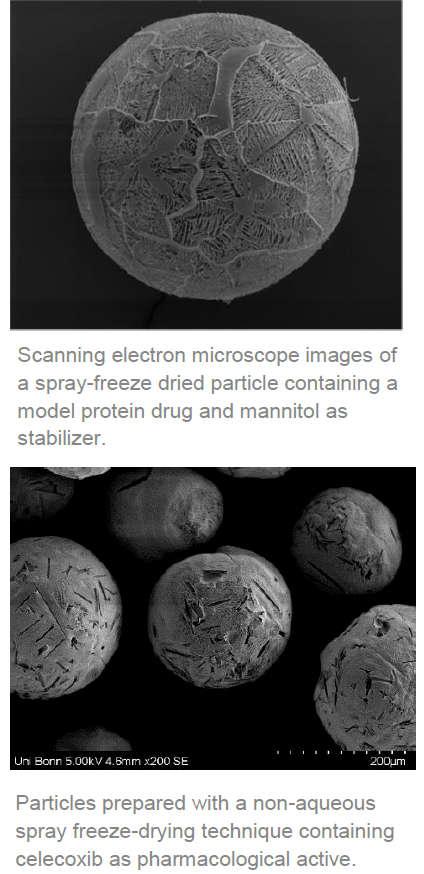
Spray freeze-drying combines the advantageous properties of the well-established techniques spray drying and freeze-drying. Thermolabile materials are dried gently due to the low temperatures, and at the same time porous, spherical microparticles with good flow properties can be produced. However, spray freeze-drying of lipophilic and poorly water-soluble compounds remains a challenge in the preparation of spray freeze-dried pharmaceutical particles. Lyophilizates produced by a classical process are often ground to powders which in turn do not exhibit good flow properties. Therefore, there is still interest in improved processes for spray freeze-drying of active pharmaceutical ingredients, in particular lipophilic compounds.
A German university has developed such an improved process. The researchers developed protocols for the spray freeze-drying of comparatively lipophilic compounds like Celecoxib, Fenofibrat, Chloramphenicol or Clotrimazol. The powder obtained by this method of spray freeze-drying is characterized by high volume, stability and sphericity of the compound particles. Therefore, the powders obtained by the process exhibit an excellent flowability and the high porosity allows for immediate dissolution when coming in contact with water. The actives are preferentially present in the spray freeze dried particles in their respective amorphous form which further increases the dissolution and can provide increased oral bioavailability.
A particular aspect of the invention is that the compound may be spray freeze-dried using simple solvent systems even without stabilizers. Yet, stabilizer or cryo-protectants may be added to the solvent system.
Besides, the adjustable low density combined with the very high sphericity is a major advantage for processing and application of the compound powder in the formulation of drugs and allows a variety of uses, such a bulk ware of actives, but also leads to the use of flowable lyophilizates applicable for drug delivery to the nose or to the deep lung.
The invention is offered to pharmaceutical companies interested in applying it to their processes for licensing. This may include further co-development depending on the partner's specifications.
Advantages and Innovations
• This is a new solution for versatile spray freeze-drying of lipophilic and hydrophilic compounds
• The solution provides powder of high sphericity with adjustable particle density that overcomes the present challenges of mechanical requirements of a powder mixture
• Better flowability at an adjustable particle size, suitable for bulk material of actives
• Better suitability for various types of drug delivery
Stage Of Development
Under development/lab tested
Stage Of Development Comment
Technology validated in lab.
Technology Readiness Level 4
Requested partner
The university offers license agreements to industrial partners
Type of partner: Pharmaceutical industry, drug formulation
Role of partner: License technology to implement it. There is a possibility co-develop further and to adjust to requirements.
Kooperationsanfrage stellen

