Internationale Partnersuche
Innovation & Technologie Angebot
Early classification of aggressive prostate cancers
Country of Origin: Germany
Reference Number: TODE20180921002
Publication Date: 11 October 2018
Summary
A German university has discovered a protein that can be used as a marker to determine the tendency of prostate tumors to metastasize. The university offers a license and research cooperation agreement.
Description
Prostate cancer is the second most common cancer in males. An estimated 1.1 million men worldwide were diagnosed with prostate cancer in 2012, accounting for 15% of the cancers diagnosed in males. The selection of the therapeutic strategy depends on the risk classification. There is a strong medical need to precisely identify those patients whose disease allows monitoring without therapeutic intervention, in order to avoid overtreatment, which comes along with high medical costs and loss of life quality.
The diagnosis, classification and therapy of prostate cancer are based on the prostate-specific antigen (PSA) test value, the Gleason score from histology obtained by prostate biopsies and an imaging diagnosis from computer tomography (CT) and bone scintigraphy. The variability of prostate cancer ranges from low-risk tumors without progression over many years to high-risk tumors with rapid local progression and high metastasis potential. There is a correlation with the risk for metastasis and the Gleason score, however there is an ongoing search for new biomarkers that directly correlate with the risk for metastasis.
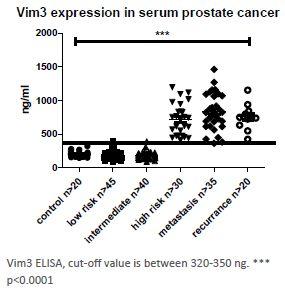
A German university discovered vimentin 3 (Vim3) as biomarker, which correlates with the risk of progression of prostate cancer. Results show high expression of Vim3 in cells of metastatic prostate and low expression in non-metastatic cancer, thus demonstrating the potential of Vim3 for risk stratification of localized prostate carcioma to enable personalized therapy planning.
Vim3 is a truncated and biologically active variant of vimentin. healthy cells express the vimentin full-length variant (Vim-fl) and are characterized by a functional cytoskeleton. If Vim-fl is no longer expressed in the cells, Vim3 is predominantly detectable. In this case the intracellular structure of the cell is disordered due to the loss of the normal cytoskeletal architecture with the consequence that cells become deformable and consequently mobile.
The university offers a license agreement to companies in the diagnostics industry, specialized in tissue detection and those specialized in detection techniques for liquids (blood, serum e.g.). The results generated in the German university still need to be validated in the form of larger studies and need a further development for clinical use. Therefore a research cooperation agreement can be included in the agreement.
Advantages and Innovations
This new marker improves the decision support of a physician regarding a therapy plan. The special feature compared to the currently used markers is that the tendency of the tumor to metastasize can be determined.
Stage Of Development
Under development/lab tested
Stage Of Development Comment
To date, 200 serum samples from prostate cancer patients, resectates and biopsies have been examined. A validation on about 800 serum samples is planned. Data on additional tumor types are available, including urethel cancer and breast cancer.
Requested partner
The university offers a license agreement to companies in the diagnostics industry, specialized in tissue detection and those specialized in detection techniques for liquids (blood, serum e.g.). The results generated in the German university still need to be validated in the form of larger studies. Therefore a research cooperation agreement can be combined with it.
If the invention is to be used as a diagnostic agent, the results generated at the university must be validated in larger studies, which will be the task of the company sought. This is where the scientists are involved (sample material, previous test procedure). It may also be necessary to develop the potential diagnostic agent into a mature product (e.g. a kit with corresponding reagents or test strips). This will probably also be done by the sought-after partner and will also involve the university.
Cooperation offer ist closed for requests

