Internationale Partnersuche
Innovation & Technologie Angebot
Efficient filling of cerebral aneurysms
Country of Origin: Germany
Reference Number: TODE20180921001
Publication Date: 8 October 2018
Summary
A German university developed an innovative coil that is used to fill the cavity of a cerebral aneurysm. The coil can be elastic and deformed in length or be inelastic and deformed in shape. This helps to lower the risks for the patients and decrease costs. The university offers a license and technical cooperation agreement.
Description
Cerebral aneurysms are a life-threatening medical event. They are often asymptomatic and are only detect as an incidental finding, e.g. during MRT (magnetic resonance tomography) of the head due to other medical indications. The rupture of the aneurysm and the consequent bleeding into the brain is the decisive health event. Half of the patients with an aneurysm rupture die, whereas almost every surviving patient has irreversible fatal brain damage leading to severe neurological deficits.
The standard operation procedure on a detected cerebral aneurysm is the catheter-based filling of the cavity with so-called embolization coils, in order to provoke thrombocyte aggregation that leads to a plaque and closes the cavity. The coils that are currently in use have a spiral shape. Depending on the aneurysm size, a large number of coils is necessary, which, in addition to increased costs, also leads to an extension of the intervention time and thus to an increased risk for the patent.
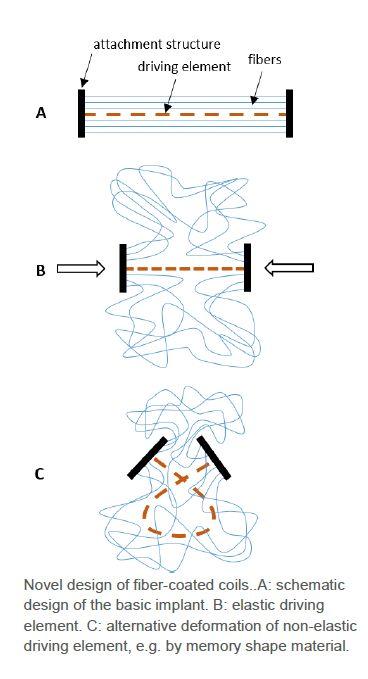
A German university developed a deformable coil, which is coated by fibers (figure A). The coil can either be elastic and deformed in length (figure B) or be inelastic and deformed in shape (figure C). In either embodiment, the coating fibers lead to a swelling of the entire device, covering a manifold higher volume than the coil alone.The invention lowers the surgical risk and increases the success rate due to the introduction of fewer devices.
The university offers a license agreement to medical technology companies that manufacture, promote and sell products for invasive surgery (end customers: clinics, surgeons / angiologists). A further development to insert the coils into the vascular in order to close the aneurysm is still pending. Therefore the university also offers a technical cooperation agreement.
Advantages and Innovations
So far there are no comparable coils on the market. The newly developed coil offers the following advantages over currently used methods:
- reduction of coils by using a self-expanding textile structure
- efficient filling of aneurysmatic cavities
- higher volume coverage
- lower surgical risk and higher success rate due to the introduction of less devices
Stage Of Development
Prototype available for demonstration
Stage Of Development Comment
Prototypes have been produced and will soon be tested in an animal model. A feasibility study on animals (probably pigs) is planned. This will be costly and time-consuming in terms of approval. Funding is currently being applied for. If a company takes up the invention and does this study, that would be good. But so far, the study is being planned.
Requested partner
The university offers a license agreement to medical technology companies that manufacture, promote and sell products for invasive surgery (end customers: clinics, surgeons / angiologists). The invention is interesting for companies offering angiography catheters, for example, which have neurotechnology/neurosurgery products in their portfolio (keyword: interventional vascular therapy).
A further development to insert the coils into the vascular in order to close the aneurysm is still pending. Therefore the university also offers a technical cooperation agreement. The potential partner would build on the positive result of a feasibility study (positive means: the concept can be implemented in vivo) and further development to insert the coils into the vascular in order to close the aneurysm, i.e. the development of the insertion system, the downscaling of the implant and the detachment unit. This would be followed by clinical studies (animal, human) to obtain approval once the clinically applicable device has been completed.
Cooperation offer ist closed for requests

