Internationale Partnersuche
Innovation & Technologie Angebot
Anti-tuberculosis drug.
Country of Origin: Germany
Reference Number: TODE20180328002
Publication Date: 28 March 2018
Summary
A German university offers lansoprazole sulfide as a drug to treat tuberculosis. The new drug is also active against multidrug-resistant tuberculosis. It has been tested in-vitro and in-vivo. Partners from the pharmaceutical industry are sought for licensing agreements.
Description
Tuberculosis resulting from infection with mycobacterium tuberculosis (Mtb) is a serious global health problem accounting for 1.7 million deaths in 2016. A major reason for the high morbidity and mortality caused by Mtb is the long duration of therapy and increasing multidrug-resistance of Mtb strains. Mtb has been treated with combination therapy for over fifty years. Yet according to the latest World Health Organization (WHO) statistics, approximately half a million new cases of multidrug-resistant tuberculosis are diagnosed every year. Of these, it is estimated that approximately 40,000 have extensively drug-resistant tuberculosis. In view of these numbers, there is a medical need for new anti-Mtb compounds to overcome resistance to known drugs in patients.
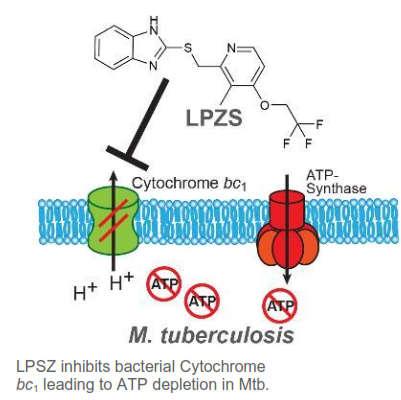
Inventors at a German university were able to demonstrate that lansoprazole and derivatives thereof exhibit growth inhibiting properties against intracellular Mtb. In particular, it has been proven that the reduced form lansoprazole sulfide is the active moiety. Yet, lansoprazole is not metabolized in a sufficient amount to serve as a prodrug. Thus, the inventors propose lansoprazole sulfide (LPZS) as a new drug to treat Mtb infections.
LPZS is a promising lead-compound which is distinct from substances identified in other TB-drug screens since its backbone structure has been an approved over-the-counter drug for the past 30 years. Thus, the finding may benefit from 40 years of intense research on pharmacological properties of pharmacology of proton pump inhibitors (PPI)-benzimidazole analogues and an extensive production pipeline maintained by several pharmaceutical companies.
The university seeks partners from the pharmaceutical industry for license agreements. These would include the rights for commercial use as well as the opportunity for further co-development.
Advantages and Innovations
This is a novel approach meeting the medical need for new compounds to overcome resistance to known drugs in patients. Lansoprazole sulfide is active against multidrug-resistant tuberculosis.
Stage Of Development
Under development/lab tested
Stage Of Development Comment
Efficacy has been proven in-vitro and in a mouse model of Mtb infection.
Requested partner
Potential licensees come from the pharmaceutical industry. They would have an interest to use the findings in their drug development activities. Joint further development could be included in the licensing agreement.
Cooperation offer ist closed for requests

