Internationale Partnersuche
Innovation & Technologie Angebot
Antibodies for anti-viral therapy.
Country of Origin: Germany
Reference Number: TODE20170516001
Publication Date: 31 May 2017
Summary
A German university has developed a procedure to stimulate antibodies that promote the anti-viral T cell response. Since only very few direct anti-viral therapeutics are available, the stimulation procedure is a valid alternative or additional therapeutic approach. The university offers a license agreement to pharmaceutical companies.
Description
Cytotoxic CD8-positive T cells constitute the crucial leukocyte subpopulation for a cellular response against viral infection. Since only very few direct anti-viral therapeutics are available, the stimulation of an efficient and specific immune response against a viral infection is a valid alternative or additional therapeutic approach. The first of such strategies has been established by use of high doses of interferon, which however has a high risk of adverse reactions of the immune system, and low response rates. Therefore, it is mandatory to search for further immune stimulatory strategies that more specifically induce an anti-viral T cell response.
The herein described approach of a German university is based on an antibody-mediated stimulation of the carcino-embryonic antigen-related cell adhesion molecule 1 (CEACAM1), which activates early parts of both the B cell and T cell receptor-induced signal transduction.
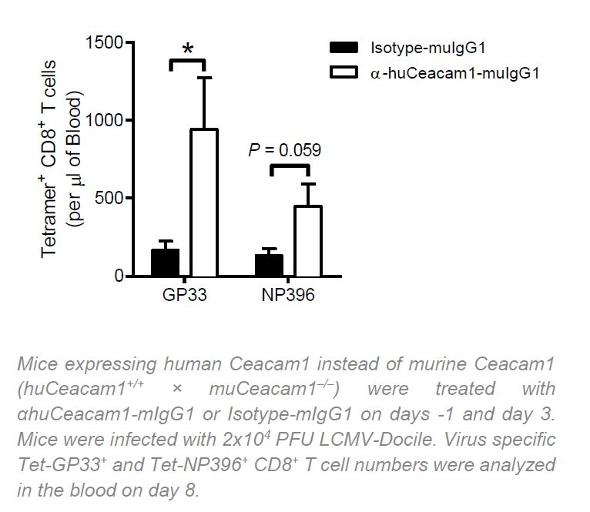
The researchers have shown on the example of the lymphocytic choriomeningitis virus (LCMV) that these antibodies stimulate the activation and expansion of virus specific cytotoxic T cells, which comes along with a reduced virus load in serum and organs of mice. In vitro studies confirmed efficacy also against the influenza virus and cytomegalovirus.
The monoclonal, humanized anti-CEACAM1 antibodies are offered under license agreement to partners from the pharmaceutical sector, who are interested in applying the method.
Advantages and Innovations
The procedure presented here is a novelty in antibody research. For the first time a therapy approach is stimulating an immune response to a viral infection. It is conceivable as a monotherapy or in combination with other anti-viral therapies.
Other advantages are:
• Applies to a broad range of viruses
• Specific immune response against specific viruses to biogas / methane without inhibition risks
Stage Of Development
Under development/lab tested
Stage Of Development Comment
The researchers have conducted experiments with a LMCV-specific immune response mouse model. Further proof of principle with the humanized variant of the antibody has been provided by means of humanized CEACAM1 mice. The antibody activates human virus-specific CD8+ T cells in vitro.
Requested partner
The university is looking for partners from the pharmaceutical industry who are interested in applying the method and is offering them a license agreement.
Cooperation offer ist closed for requests

