Internationale Partnersuche
Innovation & Technologie Angebot
Technology for reliable drug targeting to the colon
Country of Origin: Switzerland
Reference Number: TOCH20181030001
Publication Date: 30 October 2018
Summary
Scientists at a Swiss university developed a technology for colonic drug delivery by per-oral administration. The drug release is delayed until the colon is reached preventing premature release in the distal small intestine. Complete drug substance release takes place quickly in large intestinal environment assuring efficient colonic targeting. The developers seek a pharmaceutical company willing to develop and commercialize the technology. A license agreement or research cooperation is sought.
Description
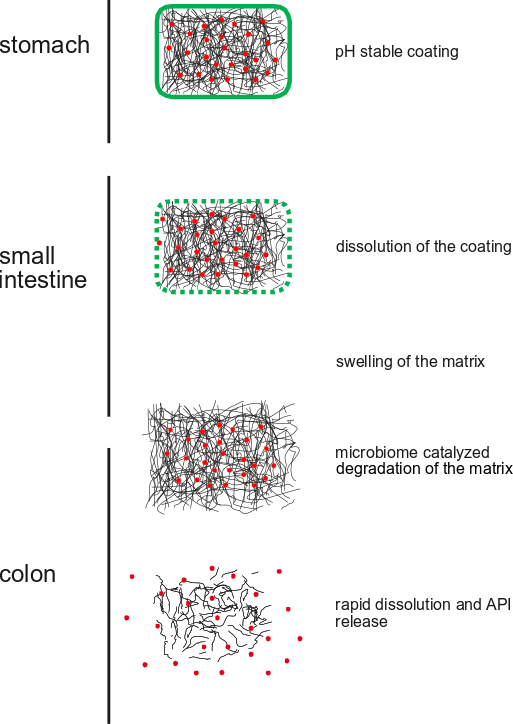
Efficient and reliable delivery of drugs to the colon has not been achieved so far, although urgently required for the treatment of inflammatory bowel disease, a malady with increasing incidence. The difficulty in consistent colonic delivery is caused by the individual and circumstantial variability of the human digestive tract (pH, residence time, water content, food intake, volume, peristaltic movement, etc.).
Scientists at a Swiss university of applied sciences have developed a novel technology for colonic drug delivery by per-oral administration.
To achieve colonic delivery coated tablets have been produced with a novel approach. The coating is a pH responsive polymer which dissolves after the gastric passage as soon as the small intestine is reached. Active pharmaceutical ingredient (API) release in the small intestine is prevented by a matrix composed of a polymeric carbohydrate. The matrix is not degraded by pancreatic enzymes and is stable in the small intestine. In contrast, rapid depolymerization of the matrix and API release take place in the large intestine triggered by catalytic activities of the microbiome in the colon.
The technology is based on a polymeric carbohydrate which has Generally Recognized as Safe (GRAS) status. The technology integrates response to two distinct signals for the specific drug release in the colon: Firstly, the pH change from stomach to small intestine and secondly the presence of a metabolically active microbiome in the colon.
Standard in vitro dissolution test carried out with tablets produced with the novel technology demonstrated that the release of the model drug 5-aminosalicylic acid (5-ASA) is delayed until the colon is reached, preventing premature release in the distal small intestine as often observed for commercially available products. Complete drug substance release took place quickly in large intestinal environment assuring efficient colonic targeting.
The developers now seek a pharmaceutical company willing to develop and commercialize the technology in the frame of a license agreement or research cooperation.
Advantages and Innovations
Advantages and innovative aspects of the proposed technology are:
- reliable colonic delivery
- less premature release in small intestine compared to conventional tablets
- reduced systemic exposure resulting in reduced side effects
- improved therapeutic drug index
- smaller API dose possible due to improved drug utilization
- GRAS status of matrix
- low cost excipients
- tablets can be produced with standard production facilities
Stage Of Development
Prototype available for demonstration
Requested partner
The specific area of activity of the partner:
- Drug development organization, pharmaceutical company.
The tasks to be performed by the partner sought:
- Pre-clinical and clinical development of the drug delivery technology.
Cooperation offer ist closed for requests

