Internationale Partnersuche
Innovation & Technologie Angebot
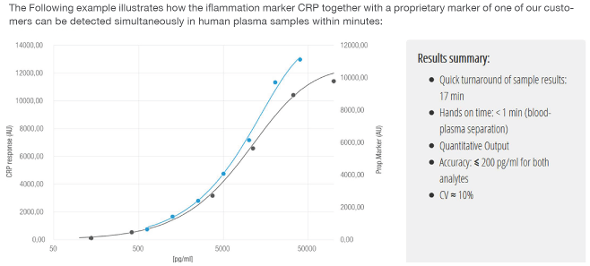
Rapid multiparameter point-of-care testing system for inflammation, allergies, cardiovascular diseases and more
Country of Origin: Austria
Reference Number: TOAT20171017001
Publication Date: 18 October 2017
Summary
An Austrian SME developed a unique testing system for automated detection of proteins in samples from human/veterinary medicine, food, environment etc. The patented technology decreases time to result by 80% compared to lab tests and offers quantification and 10 times higher sensitivity compared to other point of care tests. Up to 8 different analytes can be detected. It is also used for DNA/RNA. The SME is looking for technical/license/research cooperations & technical distribution partners.
Description
Detection of proteins in various sample material (e.g. human plasma) especially is used in human medicine for testing of inflammation, allergies, cardiovascular parameters etc. Current testing technologies are on the one hand laboratory tests such as ELISA (enzyme-linked immunosorbent assay), which are quite expensive and do not deliver quick results. On the other hand point-of-care testing methods like lateral flow immuno assays lack sensitivity and quantitative detection. In addition both technologies are not able to detect more than one parameter.
The Austrian SME successfully developed a unique testing system which enables rapid multiparameter detection (multiplexing) and quantification at the point-of-care.
The novel test system comprises:
1) an analyzer, which offers optimal ease of use by featuring:
- patented optoelectronics for highly sensitive detection
- dispensing unit for automated release of reagents from a replaceable cartridge (one cartridge for 48 tests)
- barcode module to identify test chips & patient-ID
- internal automated system check (calibration)
- higher sample rate - up to four devices can be used at the same time with one notebook
- power supply and control via USB
2) a test chip with a novel microfludic architecture (using capillary forces based flow and accelerated hybridization driven by shear forces). It contains up to 10 capture probes for different analytes and controls for RNA, DNA or proteins (reaction channel).
3) a report software, which controls the analyzer, offers intuitive user guidance through test procedure, analyses measurement data, creates individualised reports and allows optional connection to
the LIMS (laboratory information management system).
Current and potential application areas:
- The system has already been successfully applied for 5 years in dental surgeries for the detection of parodontitis (target type: RNA) and in hospitals for the detection of hospital acquired infections (target type: DNA).
- Potential new application areas are the detection of proteins in various sample material in human and veterinary medicine, food testing (e.g. detect different meat species), environmental testing (e.g. Legionella in water) etc.
- Examples for use in human medicine are testing of inflammation, allergies, cardiovascular parameters etc.
The Austrian company is looking for:
- partners from industry for the conjoint development of new applications in the fields of companion diagnostics, in vitro diagnostics, food or environmental testing, dental diagnostics (technical cooperation / license agreement).
- technical distribution partners for their system for the detection of parodontitis and/or hospital acquired infections (commercial agreement with technical assistance).
- R&D institutions / universities who would like to include the Austrian company in European R&D project proposals (research cooperation agreement)

Advantages and Innovations
The innovations of the system are:
- a novel microfluidic architecture using capillary flow and enabling accelerated hybridization
- a miniaturized dispensing unit for automated release of reagents from a replaceable cartridge
- a proprietary simple and highly sensitive optical detection system
USPs of the novel test system are:
- turnaround time (time to result) is decreased by 80% compared to ELISA tests (lab tests)
- multiplexing: up to 8 different analytes can be detected on one test chip
- compared to other point-of-care systems (lateral flow immuno assays) sensitivity is 10 times higher and quantitative results can be obtained
- automation for ease of use
Further advantages:
- easily adoptable to different diagnostic applications
- CE-IVD (in vitro diagnostics) certified; the company is ISO 13485 certified
- low initial costs
- small footprint for minimum space requirement
- integrated calibration
- automated assay procedure for minimum hands-on-time
- objective results by electronic evaluation of measurement data; detailed individual reports and statistical analysis
Stage Of Development
Already on the market
Stage Of Development Comment
The system has been successfully applied in Europe for 5 years for the detection of parodontitis and hospital acquired infections. For the detection of proteins various feasibility studies are at hand and the first application system is planned to be launched in 2018.
Requested partner
Specific area of activity of the partner:
The company is primarily looking for OEM partners from the fields of:
- pharmaceutical industry: companion diagnostics
- human medicine: in vitro diagnostics (ELISA tests, immuno assays,..)
- food testing
- environmental testing
- dental diagnostics
It is also open for cooperations with R&D institutions / universities working in the above mentioned fields.
Task to be performed by the partner:
- Technical cooperation agreement / license agreement: partners from industry for the conjoint development of new applications (e.g. transfer their ELISA test to the point-of-care; add multiplexing capabilities and/or increase sensitivity to their lateral flow immuno assay); many of the collaborations start with a feasibility study to demonstrate the great potential of the test system for an assay. The partner is expected to market the final new product. Depending on scale the Austrian company is also open to grant a license for production and marketing.
- Commercial agreement with technical assistance: the company is looking for technical distribution partners for their test system for the detection of parodontitis and/or hospital acquired infections. The partner will get technical training for the implementation and maintenance of the system.
- Research cooperation agreement: R&D institutions / universities who would like to include the Austrian company in European R&D project proposals.
Cooperation offer ist closed for requests

